A month after production of the Johnson&Johnson COVID-19 vaccine was halted for the discovery of blood clots as a rare side effect, the CDC has called an emergency meeting to go over yet another alarming situation in the Pfizer and Moderna brands.
According to the Centers for Disease Control and Prevention, they have identified at least 226 cases of myocarditis and pericarditis following the administering of the vaccine. Myocarditis is an inflammation of the heart muscle and pericarditis is an inflammation of the membrane surrounding the heart.
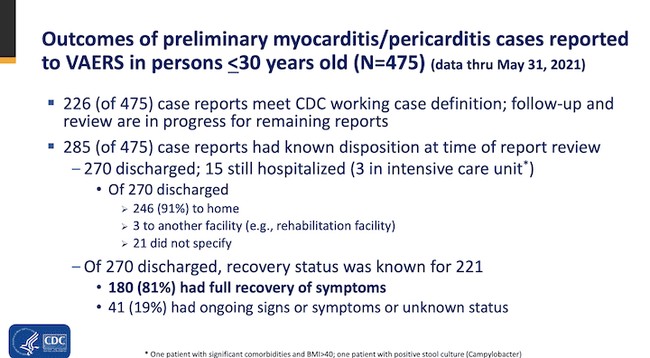
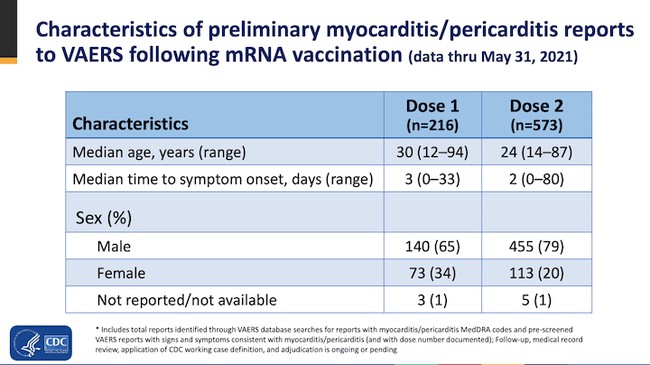
The CDC says most of those affected have recovered but 41 still had ongoing symptoms at the time of this report, with 15 hospitalized and 3 in intensive care.
Although the CDC is calling their planned meeting an “emergency” it will not convene until Friday, July 18th and will simply be added as an agenda item to a previously planned event. It will be a virtual meeting from 11a.m.ET to 5p.m. ET (times subject to change) and there is no registration required to view. The agenda for the meeting includes a discussion of the latest concerns of myocarditis and pericarditis and administering the vaccine to teens and youth, among other items.
Is this really an emergency or an abundance of caution?












Join the conversation as a VIP Member